Hydroflouric Acid
Except where otherwise noted, data are given for materials in their (at 25 °C 77 °F, 100 kPa). Hydrofluoric acid (HF) is a of in. Its chemical formula is HF.

Hydrofluoric Acid Chemical Formula
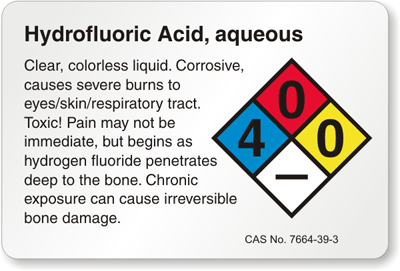
HYDROFLUORIC ACID Revision Date 18-Jan-2018 Skin Contact Wash off immediately with plenty of water for at least 15 minutes. Immediate medical attention is required. Inhalation If not breathing, give artificial respiration.
It is a weak, but very corrosive and extremely toxic. It can dissolve to make. If it gets on the skin, it can kill you. It is made by reacting with. It is used in the processing of. It is also used to make various other chemicals containing fluorine, such as (the coating in a frying pan), (the cooling agent in an ), and (used as propellants in aerosol cans).
It is used to clean metals. It is very toxic because it bonds with in the body and makes them so they cannot be used. As an acid, it can be to produce, which are also the of hydrofluoric acid. If someone gets hydrofluoric acid on them, medical attention is needed, as just small amounts are very toxic. Related pages. References.
Hydrofluoric Acid Sds
Hydrofluoric acid is used in the laboratory as an intermediate in many chemical reactions and syntheses. It serves as the fluorine source for creating many organofluorine compounds, like polytetrafluoroethylene and fluoropolymers, and for high-volume inorganic fluoride compounds such as sodium aluminum hexafluoride, cryolite, and aluminum fluoride.
Hydroflouric Acid Msds Sheet
Other uses for hydrofluoric acid include:. Removing and inhibiting rust. Extracting and refining metal, rock, brick, and oil. Producing organofluorines (fluorocarbons and refrigerants). Etching glass and silicone compounds Hydrofluoric acid can dissolve glass, so it should be stored in polyethylene containers. Because it is extremely toxic and very dangerous, users must wear personal protective equipment. Unsafe exposure can cause local and systemic injuries and can be fatal.